|
干细胞与神经再生研究组主要围绕干细胞应用研究的医学转化,重点研究全能干细胞向神经系的分化机制、人类干细胞的生物学独特性以及干细胞医药学应用的临床前研究及系统化评估等科学问题。
根据发育生物学的基本理论,我们建立了人多能干细胞定向分化至特定细胞类型的系统,并指导人胚干细胞、iPS细胞定向分化为神经系统的多种神经和胶质细胞。我们将进一步研究神经分化中细胞外信号、转录程序、转录前后表观遗传学因素如何协同指导多能干细胞向某一特定类型细胞分化;并以人多能干细胞神经定向分化系统为基础,综合利用各种技术和手段,发掘决定人类高级功能和独特性状的调控元件及调控机;同时,进一步完善规模化制备人体功能细胞的方法,为干细胞产品的临床应用奠定基础。针对严重影响人类健康的神经系统疾病,我们将建立帕金森病、亨廷顿病、中风等神经损伤及变性疾病的特色型动物模型,探讨移植细胞修复损伤的机制与影响因素。我们还将与医学、药学及材料学等领域合作进行相关细胞产品的临床试验,建立细胞产品应用于细胞替代和药物筛选的标准评估体系。

利用人胚干细胞高效地构建了大脑皮层类器官,包含有人类VZ样区域以及SVZ样区域的形态学特征(图A-B),并且有组织地发育出皮质层中存在的所有六个神经元亚型(图C-D),其具备在体大脑皮层的结构和功能特征。
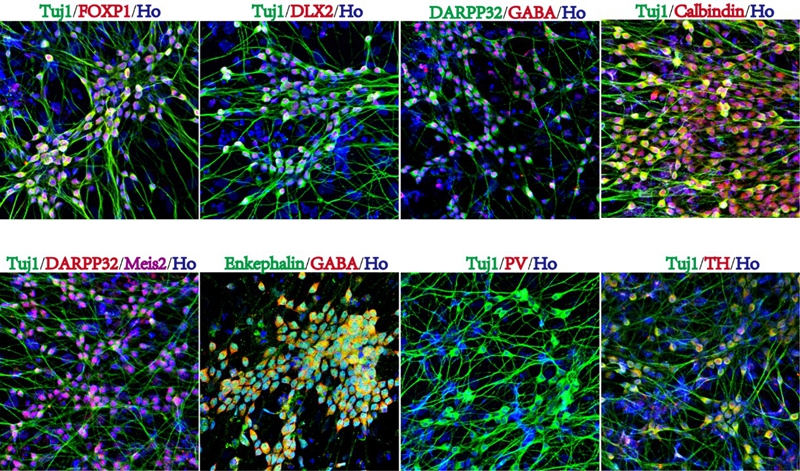
利用小分子可快速高效地从人胚胎干细胞分化获得纹状体GABA能投射神经元。

在人多能干细胞中,利用CRISPR/Cas9建立了可以靶向不同基因的基因修饰细胞系;利用标签蛋白对特定基因进行亚细胞定位;在小分子诱导下,可以对特定蛋白进行精确的剂量调控,直至完全关闭该基因蛋白的生产。
|
研究内容和目标:
人多能干细胞,包括源于最早胚胎组织的人胚干细胞与由表皮细胞等体细胞重编程而来的iPS细胞,具有分化为全身所有类型细胞的潜能,是获得各类功能细胞用于替代受损组织器官的理想细胞来源。针对当前限制基础研究向临床转化的瓶颈问题,我们将试图阐明人类细胞特异的指导多能干细胞向神经系统各类细胞分化关键信号的调控机制,从而优化多能干细胞的分化策略,高效获得所需的神经细胞类型。我们还将进一步研究正常及各种病变条件下受体组织对外源细胞的容受性,以及细胞移植入宿主后,移植细胞如何修复损伤并与周围细胞建立环路。我们还将综合讨论年龄、局部胶质增生、炎症及局部微环境对结构与功能修复能力的影响。在上述问题得以解决的前提下,我们计划与临床单位合作,选取合适病例,进行相关的临床试验。我们的最终目的是积极推动干细胞相关的研究成果向应用领域转化,指导干细胞产品应用于细胞替代治疗损伤及变性类疾病。
代表性发表论文:
- J. Liu#; Z. Hou#.; J. Wu#; K. Liu; D. Li, T. Gao; W. Liu; B. An; Y. Sun; F. Mo; L. Wang; Y. Wang*; J. Hao*; B. Hu*, Infusion of hESC derived Immunity-and-matrix regulatory cells improves cognitive ability in early-stage AD mice. Cell
Prolif 2021, 54 (8), e13085. DOI: 10.1111/cpr.13085.
- J. Wu#; D. Song#; Z. Li#; B. Guo#; Y. Xiao; W. Liu, L. Liang; C. Feng; T. Gao, Y. Chen; Y. Li, Z. Wang, J. Wen, S. Yang, P. Liu, L. Wang, Y. Wang; L. Peng; G. N. Stacey; Z. Hu, G. Feng; W. Li; Y. Huo; R. Jin, N. Shyh-Chang, Q. Zhou, L. Wang;
B. Hu*; H. Dai*; J. Hao*, Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res 2020, 30 (9), 794-809. DOI: 10.1038/s41422-020-0354-1
- W. Zhu#, B. Zhang#, M. Li#, F. Mo, T. Mi, Y. Wu, Z. Teng, Q. Zhou*, W. Li*, and B. Hu*, Precisely controlling endogenous protein dosage in hPSCs and derivatives to model FOXG1 syndrome. Nat Commun, 2019. 10(1): p. 928.
DOI: 10.1038/s41467-019-08841-7
- Y.P. Xu#, Y. Qiu#, B. Zhang#, G. Chen#, Q. Chen, M. Wang, F. Mo, J. Xu, J. Wu, R.R. Zhang, M.L. Cheng, N.N. Zhang, B. Lyu, W.L. Zhu, M.H. Wu, Q. Ye, D. Zhang, J.H. Man, X.F. Li, J. Cui, Z. Xu, B. Hu*, X. Zhou*, and C.F. Qin*, Zika virus
infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res, 201 9. 29(4): p. 265-273. DOI: 10.1038/s41422-019-0152-9
- W. Zhu, M. Li, Y. Wu, and B. Hu*, Precise immune tolerance for hPSC derivatives in clinical application. Cell Immunol, 2018. 326: p. 15-23. DOI: 10.1016/j.cellimm.2017.08.005
- W. Zhang#, H. Wan#, G. Feng#, J. Qu#, J. Wang, Y. Jing, R. Ren, Z. Liu, L. Zhang, Z. Chen, S. Wang, Y. Zhao, Z. Wang, Y. Yuan, Q. Zhou, W. Li*, G.H. Liu*, and B. Hu*, SIRT6 deficiency results in developmental retardation in cynomolgus
monkeys. Nature, 2018. 560(7720): p. 661-665. DOI: 10.1038/s41586-018-0437-z
- B. Zhang#, Y. He#, Y. Xu#, F. Mo, T. Mi, Q.S. Shen, C. Li, Y. Li, J. Liu, Y. Wu, G. Chen, W. Zhu, C. Qin*, B. Hu*, and G. Zhou*, Differential antiviral immunity to Japanese encephalitis virus in developing cortical organoids. Cell Death Dis,
2018. 9(7): p. 719. DOI: 10.1038/s41419-018-0763-y
- M. Wu, D. Zhang, C. Bi, T. Mi, W. Zhu, L. Xia, Z. Teng, B. Hu*, and Y. Wu*, A Chemical Recipe for Generation of Clinical-Grade Striatal Neurons from hESCs. Stem Cell Reports, 2018. 11(3): p. 635-650. DOI: 10.1016/j.stemcr.2018.08.005
- Y. Wang#, W. Zhu#, M. Wu#, Y. Wu, Z. Liu, L. Liang, C. Sheng, J. Hao, L. Wang, W. Li, Q. Zhou*, B. Hu*, Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson's
Disease. Stem Cell Reports, 2018. 11(1): p. 171-182. DOI: 10.1016/j.stemcr.2018.05.010
- X. Wang#, T. Li#, T. Cui#, D. Yu#, C. Liu#, L. Jiang, G. Feng, L. Wang, R. Fu, X. Zhang, J. Hao, Y. Wang, L. Wang, Q. Zhou*, W. Li*, B. Hu*, Human embryonic stem cells contribute to embryonic and extraembryonic lineages in mouse embryos
upon inhibition of apoptosis. Cell Res, 2018. 28(1): p. 126-129. DOI: 10.1038/cr.2017.138
- Z. Li#, L. Wang#, L. Wang#, G. Feng#, X. Yuan#, C. Liu, K. Xu, Y. Li, H. Wan, Y. Zhang, Y.F. Li, X. Li, W. Li*, Q. Zhou*, B. Hu*, Generation of Bimaternal and Bipaternal Mice from Hypomethylated Haploid ESCs with Imprinting Region Deletions. Cell Stem Cell,
2018. 23(5): p. 665-676 e4. DOI: 10.1016/j.stem.2018.09.004
- L. Yuan#, X. Huang#, Z. Liu#, F. Zhang#, X. Zhu#, J.Y. Yu#, X. Ji, Y.P. Xu, G. Li, C. Li, H.J. Wang, Y. Deng, M. Wu, M. Cheng, Q. Ye, D. Xie, X. Li, X. Wang, W. Shi, B. Hu, P. Shi, Z. Xu*, C. Qin*, A single mutation in the prM protein
of Zika virus contributes to fetal microcephaly. Science, 2017. 358(6365): p. 933-936. DOI: 10.1126/science.aam7120
- K. Qian#, H. Huang#, A. Peterson, B. Hu, N.J. Maragakis, G. Ming, H. Chen*, S. Zhang*, Sporadic ALS Astrocytes Induce Neuronal Degeneration In Vivo. Stem Cell Reports, 2017. 8(4): p. 843-855. DOI: 10.1016/j.stemcr.2017.03.003
- T. Li#, G. Feng#, Y. Li#, M. Wang#, J. Mao, J. Wang, X. Li, X. Wang, B. Qu, L. Wang, X. Zhang, H. Wan, T. Cui, C. Wan, L. Liu, X. Zhao, B. Hu, W. Li, and Q. Zhou*, Rat embryonic stem cells produce fertile offspring through
tetraploid complementation. Proc Natl Acad Sci U S A, 2017. 114(45): p. 11974-11979. DOI: 10.1073/pnas.1708710114
- Z. Li#, H. Wan#, G. Feng#, L. Wang#, Z. He, Y. Wang, X. Wang, W. Li*, Q. Zhou*, B. Hu*, Birth of fertile bimaternal offspring following intracytoplasmic injection of parthenogenetic haploid embryonic stem cells. Cell Res,
2016. 26(1): p. 135-8. DOI: 10.1038/cr.2015.151
- Y. Jiang#, M. Du#, M. Wu, Y. Zhu, X. Zhao, X. Cao, X. Li, P. Long, W. Li*, B. Hu*, Phosphatidic Acid Improves Reprogramming to Pluripotency by Reducing Apoptosis. Stem Cells Dev, 2016. 25(1): p. 43-54. DOI: 10.1089/scd.2015.0159
- L. Gao, Y. Zhang, B. Hu, J. Liu, P. Kong, S. Lou, Y. Su, T. Yang, H. Li, Y. Liu, C. Zhang, L. Gao, L. Zhu, Q. Wen, P. Wang, X. Chen, J. Zhong, and X. Zhang*, Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy
and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. J Clin Oncol, 2016. 34(24): p. 2843-50. DOI: 10.1200/JCO.2015.65.3642
- H. Chen*, K. Qian, W. Chen, B. Hu, L.W.t. Blackbourn, Z. Du, L. Ma, H. Liu, K.M. Knobel, M. Ayala, and S.C. Zhang*, Human-derived neural progenitors functionally replace astrocytes in adult mice. J Clin Invest, 2015.
125(3): p. 1033-42. DOI: 10.1172/JCI69097
- L. Ma, B. Hu, Y. Liu, S.C. Vermilyea, H. Liu, L. Gao, Y. Sun, X. Zhang, S. Zhang*, Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell, 2012. 10(4):
p. 455-64. DOI: 10.1016/j.stem.2012.01.021
- B. Hu and S. Zhang, Directed Differentiation of Neural-stem cells and Subtype-Specific Neurons from hESCs, in Cellular Programming and Reprogramming: Methods and Protocols, S. Ding, Editor. 2010, Humana Press: Totowa,
NJ. p. 123-137.
- B. Hu, J. Weick, J. Yu, L. Ma, X. Zhang, J. Thomson, and S. Zhang*, Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A,
2010. 107(9): p. 4335-40. DOI: 10.1073/pnas.0910012107
- B. Hu and S. Zhang*, Differentiation of spinal motor neurons from pluripotent human stem cells. Nat Protoc, 2009. 4(9): p. 1295-304. DOI: 10.1038/nprot.2009.127
- B. Hu, Z. Du, and S. Zhang*, Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc, 2009. 4(11): p. 1614-22. DOI: 10.1038/nprot.2009.186
- B. Hu#, Z. Du#, X. Li, M. Ayala, and S. Zhang*, Human oligodendrocytes from embryonic stem cells: conserved SHH signaling networks and divergent FGF effects. Development, 2009. 136(9): p. 1443-52. DOI: 10.1242/dev.029447
|

