| 我们实验室主要以小鼠为模型,综合利用遗传学、胚胎培养、胚胎干细胞、分子生物学和生物化学等技术,研究动物早期胚胎发育和生殖细胞形成的分子机制。 研究方向 1) 早期胚胎细胞谱系形成分子机制:在胚胎发育过程中,全能的受精卵经过分化成多能性细胞,后进一步发育形成具有命运不同的各种胚层的细胞。最近,我们建立了高效的哺乳动物早期原肠胚体外研究模型,同时建立了第一个哺乳动物原肠运动即将起始的活化态多能干细胞(fPSCs)。目前,我们正在利用这些模型研究哺乳动物早期胚胎细胞谱系形成中的遗传和表观遗传机制。 2) 早期胚胎发育母源调控机制研究:合子基因组激活以前,胚胎发育主要受到母源效应基因调控。近期,我们鉴定了哺乳动物卵母细胞和早期胚胎特异表达的蛋白复合体SCMC,该复合体是哺乳动物早期胚胎发育必需。目前,正在解析蛋白复合体SCMC的作用机制;此外,还研究母源DNA损伤修复及母源RNA降解在早期胚胎发育中的分子机制。 3) 哺乳动物生殖细胞形成的分子机制:最近我们发现Pre-mRNA选择性剪接因子在生殖细胞形成过程中特异性高表达,并具有重要功能。目前,我们主要利用基因敲除小鼠和RNA测序等技术,研究Pre-mRNA选择性剪接调控生殖细胞形成的作用机制。 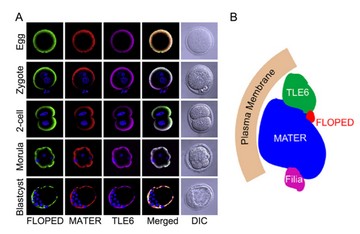
SCMC在卵母细胞及早期胚胎的分布(A)及模式图(B) (Li et al., Dev. Cell, 2008)
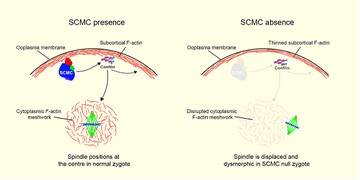
SCMC通过F-actin细胞骨架调控受精卵的均等分裂 (Yu et al., Nat. Commun., 2014)
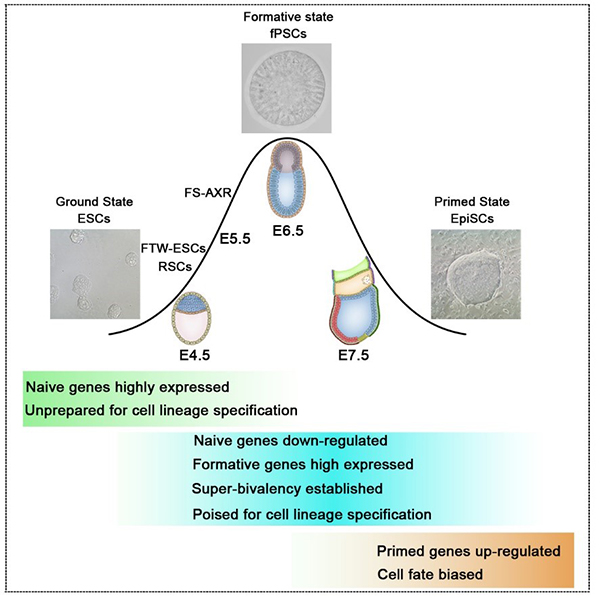
Formative pluripotent stem cells (fPSCs), (Wang et al., Cell Res, 2021)
| 研究内容和目标:
我们实验室主要研究哺乳动物配子细胞中特异表达的关键因子在早期胚胎发育和生殖细胞形成中的作用机制,同时研究哺乳动物早期胚胎细胞谱系分化的机制,我们的研究结果不仅可以为人类生殖疾病和出生缺陷的预防和治疗参考,同时还可为无副作用避孕药物的研发提供潜在靶点。 代表性发表论文(*通讯作者论文): - Chi Q, Ou G, Qin D, Han Z, Li J, Xiao Q, Gao Z, Xu C, Qi Q, Liu Q, Liu S, Li J, Guo L, Lu Y, Chen J, Wang X, Shi H, Li L*, Deng D*. Structural basis of the subcortical maternal complex and its implications in reproductive disorders. Nature Structural & Molecular Biology. 2024 Jan;31(1):115-124. doi: 10.1038/s41594-023-01153-x. Epub 2024 Jan 4
- Nie X, Xu Q, Xu C, Chen F, Wang Q, Qin D, Wang R, Gao Z, Lu X, Yang X, Wu Y, Gu C, Xie W*, Li L*. Maternal TDP-43 interacts with RNA Pol II and regulates zygotic genome activation. Nature Communications. 2023 Jul 17 (14: 4275). https://doi.org/10.1038/s41467-023-39924-1.
- Quan Y, Wang M, Xu C, Wang X, Qin D, Lin Y, Lu X, Lu F*, Li L*. Cnot8 eliminates naïve regulation networks and is essential for naïve-to-formative pluripotency transition. Nucleic Acids Research. 2022 May 6;50(8):4414-4435. doi: 10.1093/nar/gkac236.
- Xiong Z, Xu K, Lin Z, Kong F, Wang Q, Quan Y, Sha Q, Li F, Zou Z, Liu L, Ji S, Chen Y, Zhang H, Fang J, Yu G, Liu B, Wang L, Wang H, Deng H, Yang X, Fan H, Li L*, Xie W*. Ultrasensitive Ribo-seq reveals translational landscapes during oocyte-to-embryo transition and early development. Nature Cell Biology. 2022. doi:10.1038/s41556-022-00928-6.
- Wang X, Xiang Y, Yu Y, Wang R, Zhang Y, Xu Q, Sun H, Zhao Z, Jiang X, Wang X, Lu X, Qin D, Quan Y, Zhang J, Shyh-Chang N, Wang H, Jing N, Xie W*, Li L*. Formative pluripotent stem cells show features of epiblast cells poised for gastrulation. Cell Research. 2021 May; 31(5): 526-541. doi.org/10.1038/s 41422-021-00477-x.
- Ma H, Zhai J, Wan H, Jiang X, Wang X, Wang L, Xiang Y, He X, Zhao Z, Zhao B, Zheng P*, Li L*, Wang H*. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019 Oct 31. pii: eaax7890. doi: 10.1126/science.aax7890.
- Qin D, Gao Z, Xiao Y, Zhang X, Ma H, Yu X, Nie X, Fan N, Wang X, OuYang Y, Sun Q, Yi Z, Li L*. The subcortical maternal complex protein Nlrp4f is involved in cytoplasmic lattice formation and organelle distribution. Development. 2019 Oct 18; 146(20). pii: dev183616. doi: 10.1242/dev.183616.
- Lu X, Gao Z, Qin D, Li L*. A maternal functional module in the mammalian oocyte-to-embryo transition. Trends in Molecular Medicine. 2017 Nov; 23 (11) 1014–1023.
- Liu W, Wang F, Xu Q, Shi J, Zhang X, Lu X, Gao Z, Ma H, Zhao Z, Duan E, Gao F, Gao S*, Yi Z*, Li L*. BCAS2 is involved in alternative mRNA splicing in spermatogonia and the transition to meiosis. Nature Communications. 2017 Jan 27; 8:14182.
- Xu Q, Wang F, Xiang Y, Zhang X, Zhao Z, Gao Z, Liu W, Lu X, Liu Y, Yu X, Wang H, Huang J, Yi Z, Gao S*, Li L*. Maternal BCAS2 protects genomic integrity in mouse early embryonic development. Development. 2015 Nov 15; 142 (22):3943-53.
- Yu X, Yi Z, Gao Z, Qin D, Zhai Y, Chen X, Ou-Yang Y, Wang Z, Zheng P, Zhu M, Wang H, Sun QY, Dean J*, Li L*. The subcortical maternal complex controls symmetric division of mouse zygotes by regulating F-actin dynamics. Nature Communications. 2014 Sep 11; 5:4887.
- Li L*, Baibakov B and Dean J. A subcortical maternal complex essential for pre-implantation mouse embryogenesis. Developmental Cell. 2008 Sep; 15(3): 416-25.

|

